Define Calorimeter Constant . Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The heat of reaction is important for understanding the. For example, when an exothermic. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. What is the calorimeter constant? A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known.
from www.chegg.com
It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. What is the calorimeter constant? Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. For example, when an exothermic. The heat of reaction is important for understanding the. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
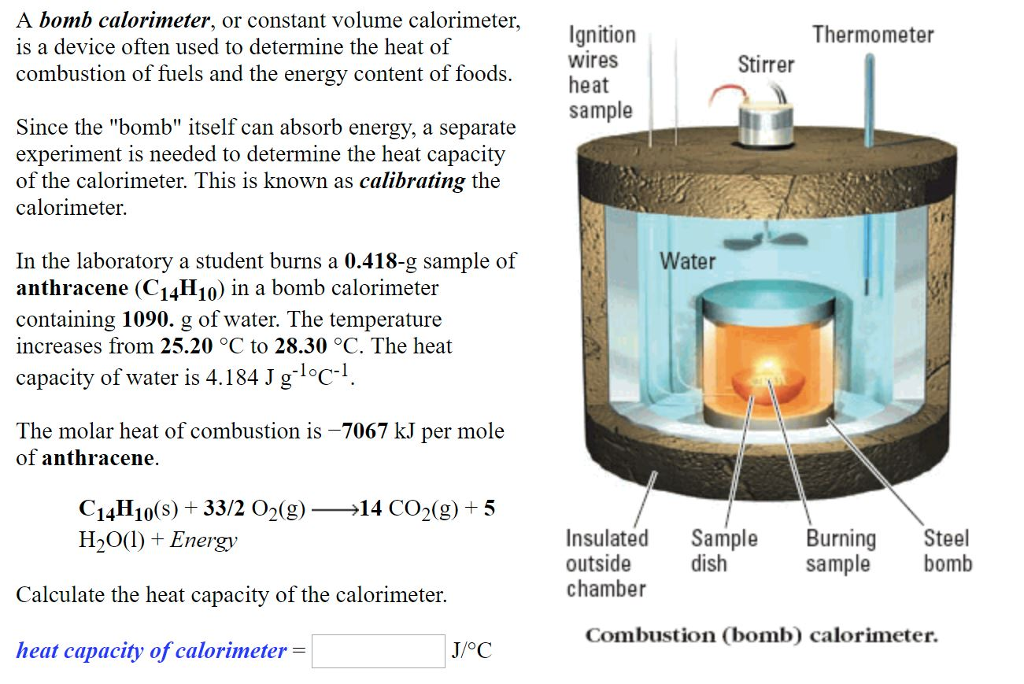
Solved A bomb calorimeter, or constant volume calorimeter,
Define Calorimeter Constant A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. What is the calorimeter constant? Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. The heat of reaction is important for understanding the. It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real.
From askfilo.com
In a constant pressure calorimeter with heat capacity of 453 Jk−1,200 mL Define Calorimeter Constant It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. The heat of reaction is important for understanding the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics. Define Calorimeter Constant.
From studylib.net
U3 S1 L3 calorimetry Define Calorimeter Constant A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is therefore necessary to calibrate the. Define Calorimeter Constant.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Define Calorimeter Constant Apply the first law of thermodynamics to calorimetry. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. What is the calorimeter constant? A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. The heat of. Define Calorimeter Constant.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Define Calorimeter Constant A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. We need to find the difference between the heat lost by the hot water when it droped from 60.0. Define Calorimeter Constant.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Define Calorimeter Constant What is the calorimeter constant? Apply the first law of thermodynamics to calorimetry. It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat of reaction is important for. Define Calorimeter Constant.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Define Calorimeter Constant For example, when an exothermic. It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. The heat of reaction is important for understanding the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Define Calorimeter Constant.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Define Calorimeter Constant For example, when an exothermic. What is the calorimeter constant? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. Compare heat flow from hot. Define Calorimeter Constant.
From users.highland.edu
Calorimetry Define Calorimeter Constant What is the calorimeter constant? The heat of reaction is important for understanding the. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law. Define Calorimeter Constant.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Define Calorimeter Constant Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. What is the calorimeter constant? We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It is therefore necessary to calibrate the. Define Calorimeter Constant.
From www.chegg.com
Calculate the calorimeter constant (From Lab) So for Define Calorimeter Constant A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. What is the calorimeter constant? It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. Apply the first law of thermodynamics to calorimetry. We need to find the difference between. Define Calorimeter Constant.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Define Calorimeter Constant What is the calorimeter constant? Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat of reaction is important for understanding the. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. It. Define Calorimeter Constant.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Define Calorimeter Constant Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. The heat of reaction is important for understanding the. What is the calorimeter constant? It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. For example, when an exothermic. A container. Define Calorimeter Constant.
From www.thoughtco.com
Calorimeter Definition in Chemistry Define Calorimeter Constant It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. A container that prevents heat transfer. Define Calorimeter Constant.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Define Calorimeter Constant For example, when an exothermic. It is therefore necessary to calibrate the calorimeter by measuring the temperature change that results from the introduction of a known. What is the calorimeter constant? Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in. Define Calorimeter Constant.
From www.reddit.com
How exactly is this calorimeter an example of constantpressure Define Calorimeter Constant Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The heat of reaction is important for understanding the. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to. Define Calorimeter Constant.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Define Calorimeter Constant Calorimeters measure the heat of a chemical reaction or a physical change like ice melting to liquid water. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped from. Define Calorimeter Constant.
From study.com
Calorimetry Definition, Equation & Types Lesson Define Calorimeter Constant A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Apply the first law of thermodynamics to calorimetry. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. It is therefore necessary to calibrate. Define Calorimeter Constant.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Define Calorimeter Constant A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to. A calorimeter is a device used to measure the amount of heat involved in. Define Calorimeter Constant.